Procedure
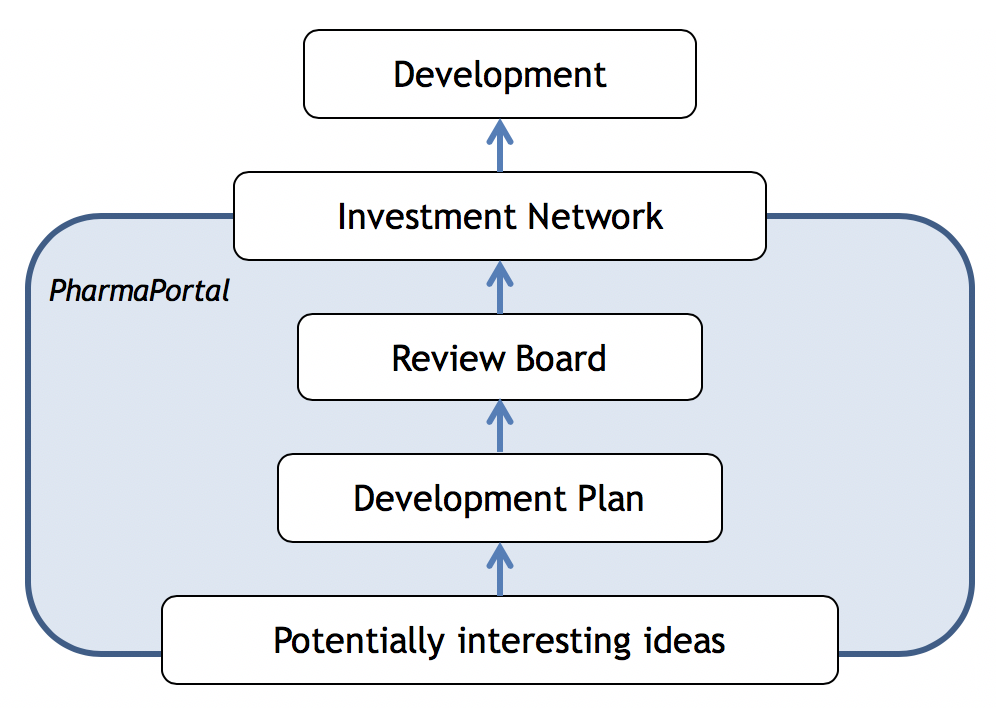
Anybody from the participating partners can submit ideas to the Pharma Portal. These will be screened for opportunity. A positive initial quick scan is followed by an in-depth analysis with structured assessment of the target, role in pathology, medical need, evidence and data generated so far, available tools, competition, intellectual property, chances and risks.
The screening and analyses will be done in close collaboration with the idea-owner and the idea-owner will be provided with critical feedback and support to bring the development plan to a higher level. The resulting development plan, including milestones, resources, costs and timelines, will be presented to the Pharma Portal Review Board for evaluation and approval. The Board will evaluate each individual project and development plan for quality, societal impact and commercial perspective.
The Pharma Portal is positioned to speed up the development and to improve the chance of obtaining funding by providing access to investors, willing to invest in initiatives approved by the Pharma Portal Review Board.
Pharma Portal BV, chamber of commerce KVK 73046027
L.J. Zielstraweg 2, 9713 GX Groningen, The Netherlands
Privacy Statement